Source: new energy leader, by
Abstract: at present, the lithium salts in commercial lithium-ion battery electrolyte are mainly LiPF6 and LiPF6 have given the electrolyte excellent electrochemical performance, but LiPF6 has poor thermal and chemical stability, and is very sensitive to water.
At present, the lithium salts in commercial lithium-ion battery electrolyte are mainly LiPF6 and LiPF6 have given the electrolyte excellent electrochemical performance. However, LiPF6 has poor thermal and chemical stability, and is very sensitive to water. Under the action of a small amount of H2O, acid substances such as HF will be decomposed, and then the positive material will be corroded, and the transition metal elements will be dissolved, and the surface of negative electrode will be migrated to destroy SEI film, The results show that the SEI film continues to grow, which leads to the continuous decline of the capacity of lithium-ion batteries.
In order to overcome these problems, people have hoped that the lithium salts of imide with more stable H2O and better thermal and chemical stability, such as lithium salts such as LiTFSI, lifsi and liftfsi, are limited by cost factors and the anions of lithium salts such as LiTFSI can not be solved for corrosion of Al foil, etc., LiTFSI lithium salt has not been applied in practice. Recently, VARVARA sharova of German HIU laboratory has found a new way for the application of imide lithium salts as electrolyte additives.
The low potential of graphite negative electrode in Li-ion battery will lead to the decomposition of electrolyte on its surface, forming passivation layer, which is called SEI film. SEI film can prevent electrolyte from decomposition on the negative surface, so the stability of SEI film has a crucial influence on the cycle stability of lithium-ion batteries. Although lithium salts such as LiTFSI can not be used as solute of commercial electrolyte for a while, it has been used as additives and has achieved very good results. VARVARA sharova experiment found that adding 2wt% LiTFSI in the electrolyte can effectively improve the cycle performance of lifepo4/ graphite battery: 600 cycles at 20 ℃ and the capacity decline is less than 2%. In the control group, the electrolyte with 2wt% VC additive is added. Under the same conditions, the decline of the capacity of the battery reaches about 20%.
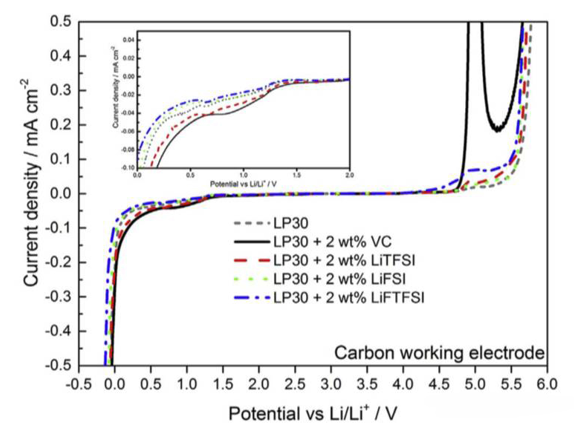
In order to verify the effect of different additives on the performance of lithium-ion batteries, the blank group lp30 (EC: DMC = 1:1) without additives and the experimental group with VC, LiTFSI, lifsi and liftfsi were prepared by varvarvara sharova respectively. The performance of these electrolytes was evaluated by button half cell and full cell.
The figure above shows the voltammetric curves of the electrolytes of the blank control group and the experimental group. During the reduction process, we noticed that an obvious current peak appeared in the electrolyte of the blank group at about 0.65v, corresponding to the reduction decomposition of EC solvent. The decomposition current peak of the experimental group with VC additive shifted to the high potential, which was mainly because the decomposition voltage of VC additive was higher than that of EC, Therefore, the decomposition occurred first, which protected EC. However, the voltammetric curves of the electrolyte added with LiTFSI, lifsi and littfsi additives were not significantly different from those of the blank group, which indicated that the imide additives could not reduce the decomposition of EC solvent.
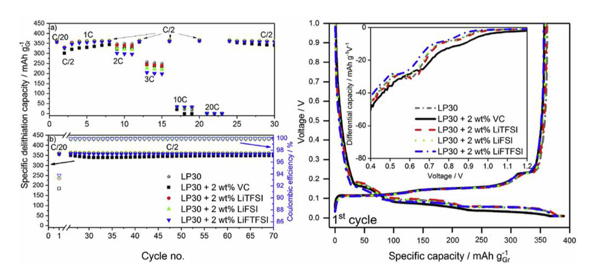
The figure above shows the electrochemical performance of graphite anode in different electrolytes. From the efficiency of first charge and discharge, the coulomb efficiency of blank group is 93.3%, the first efficiency of electrolytes with LiTFSI, lifsi and liftfsi are 93.3%, 93.6% and 93.8%, respectively. However, the first efficiency of electrolytes with VC additive is only 91.5%, which is mainly because during the first lithium intercalation of graphite, VC decomposes on the surface of graphite anode and consumes more Li.
The composition of SEI film will have a great influence on the ionic conductivity, and then affect the rate performance of Li ion battery. In the rate performance test, it is found that the electrolyte with lifsi and liftfsi additives has a slightly lower capacity than other electrolytes in high current discharge. In the C / 2 cycle test, the cycle performance of all the electrolytes with imide additives is very stable, while the capacity of the electrolytes with VC additives decreases.
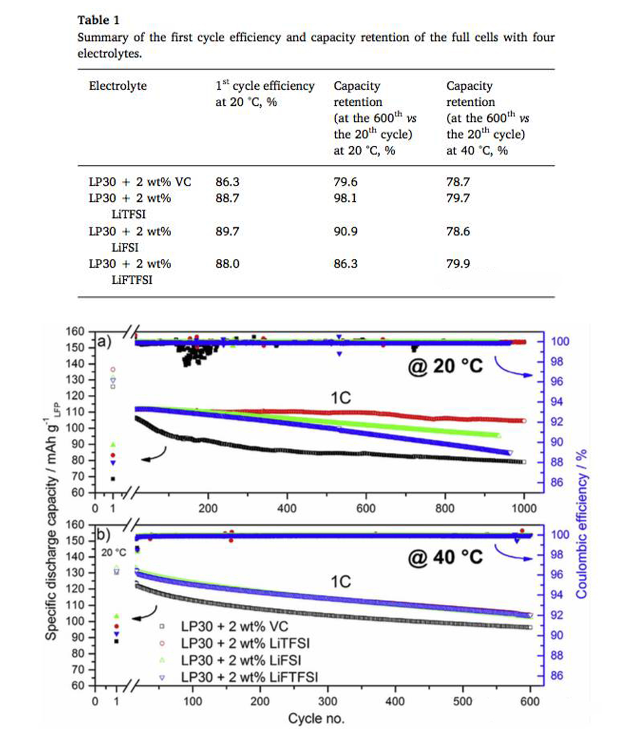
In order to evaluate the stability of electrolyte in the long-term cycle of lithium-ion battery, VARVARA sharova also prepared LiFePO4 / graphite full cell with button cell, and evaluated the cycle performance of electrolyte with different additives at 20 ℃ and 40 ℃. The evaluation results are shown in the table below. It can be seen from the table that the efficiency of the electrolyte with LiTFSI additive is significantly higher than that with VC additive for the first time, and the cycling performance at 20 ℃ is even more overwhelming. The capacity retention rate of the electrolyte with LiTFSI additive is 98.1% after 600 cycles, while the capacity retention rate of the electrolyte with VC additive is only 79.6%. However, this advantage disappears when the electrolyte is cycled at 40 ℃, and all electrolytes have similar cycling performance.
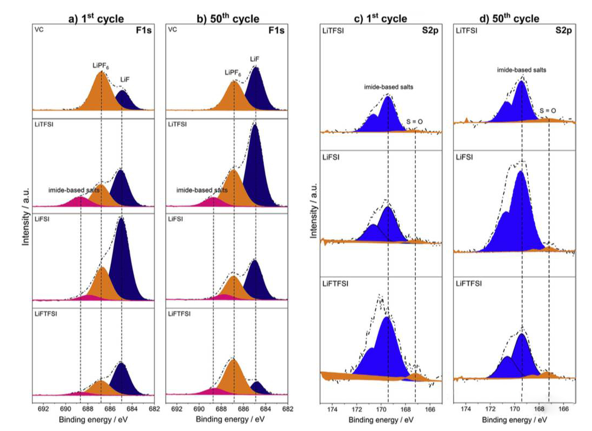
From the above analysis, it is not difficult to see that the cycle performance of lithium-ion battery can be significantly improved when lithium imide salt is used as electrolyte additive. In order to study the action mechanism of additives such as LiTFSI in lithium-ion batteries, VARVARA sharova analyzed the composition of SEI film formed on the surface of graphite anode in different electrolytes by XPS. The following figure shows the XPS analysis results of SEI film formed on the surface of graphite anode after the first and the 50th cycles. It can be seen that the LIF content in the SEI film formed in the electrolyte with LiTFSI additive is significantly higher than that in the electrolyte with VC additive. Further quantitative analysis of the composition of SEI film shows that the order of LIF content in SEI film is lifsi > liftfsi > LiTFSI > VC > blank group after the first cycle, but the SEI film is not invariable after the first charge. After 50 cycles, the LIF content of SEI film in lifsi and liftfsi electrolyte decreased by 12% and 43%, respectively, while the LIF content of electrolyte added with LiTFSI increased by 9%.
Generally, we think that the structure of the SEI membrane is divided into two layers: the inner inorganic layer and the outer organic layer. The inorganic layer is mainly composed of LIF, Li2CO3 and other inorganic components, which have better electrochemical performance and higher ionic conductivity. The outer organic layer is mainly composed of porous electrolyte decomposition and polymerization products, such as roco2li, PEO and so on, which has no strong protection for the electrolyte, Therefore, we hope that the SEI membrane contains more inorganic components. Imide additives can bring more inorganic LIF components to the SEI membrane, which makes the structure of the SEI membrane more stable, can better prevent electrolyte decomposition in the battery cycle process, reduce Li consumption, and significantly improve the cycle performance of the battery.
As electrolyte additives, especially LiTFSI additives, imide lithium salts can significantly improve the cycle performance of the battery. This is mainly due to the fact that the SEI film formed on the surface of graphite anode has more LIF, thinner and more stable SEI film, which reduces the decomposition of electrolyte and reduces the interface resistance. However, from the current experimental data, LiTFSI additive is more suitable for use at room temperature. At 40 ℃, LiTFSI additive has no obvious advantage over VC additive.
Post time: Apr-15-2021
